A new -study report, conducted by HYDRA Marine Sciences and commissioned by Holland Bioplastics concludes that PLA does not lead to the creation of persistent microplastics in the environment.
In contrast to non-biodegradable, fossil-based polymers, polylactic acid (PLA) has the ability to undergo hydrolysis in the presence of water or humidity. This process breaks down PLA into progressively smaller molecules, eventually rendering it soluble in water. As long as moisture or humidity remains, PLA objects and fragments continue to decrease in size, ultimately breaking down into oligomers and monomers, primarily lactic acid. These smaller components of PLA, once fully hydrolyzed, do not persist as nano- or microplastics. Instead, they are biodegradable and are further broken down by microorganisms, which use them as sources of carbon for energy and biomass production. The final stage of this process is the mineralization of the original polymer carbon into biomass, carbon dioxide, methane, and water, effectively eliminating the potential for lasting microplastic pollution.
This research highlights PLA’s potential as a sustainable and eco-friendly material, offering a viable solution to the global challenge of reducing plastic pollution. By choosing PLA, industries and consumers can take a significant step toward a greener, more sustainable future.
For more detailed information, you can view the full press release here.
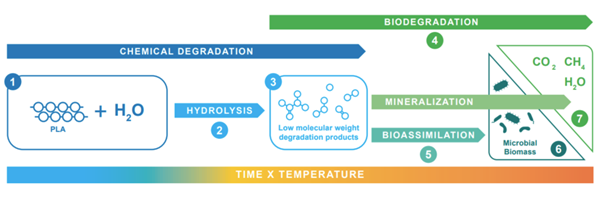
Mechanisms for PLA Degradation: In the presence of water (1), PLA undergoes hydrolysis (2) as a pure chemical process of polymer degradation during which low molecular weight intermediates (3) such as oligomers and lactic acid monomers are produced. These become soluble and can be biodegraded (4). Microbes take up these oligomers and monomers as food (5) and use them to build up biomass (6) and as energy for bolism. Ultimately, this leads to mineralization (7) of the original polymer carbon into carbon dioxide, methane, and water

